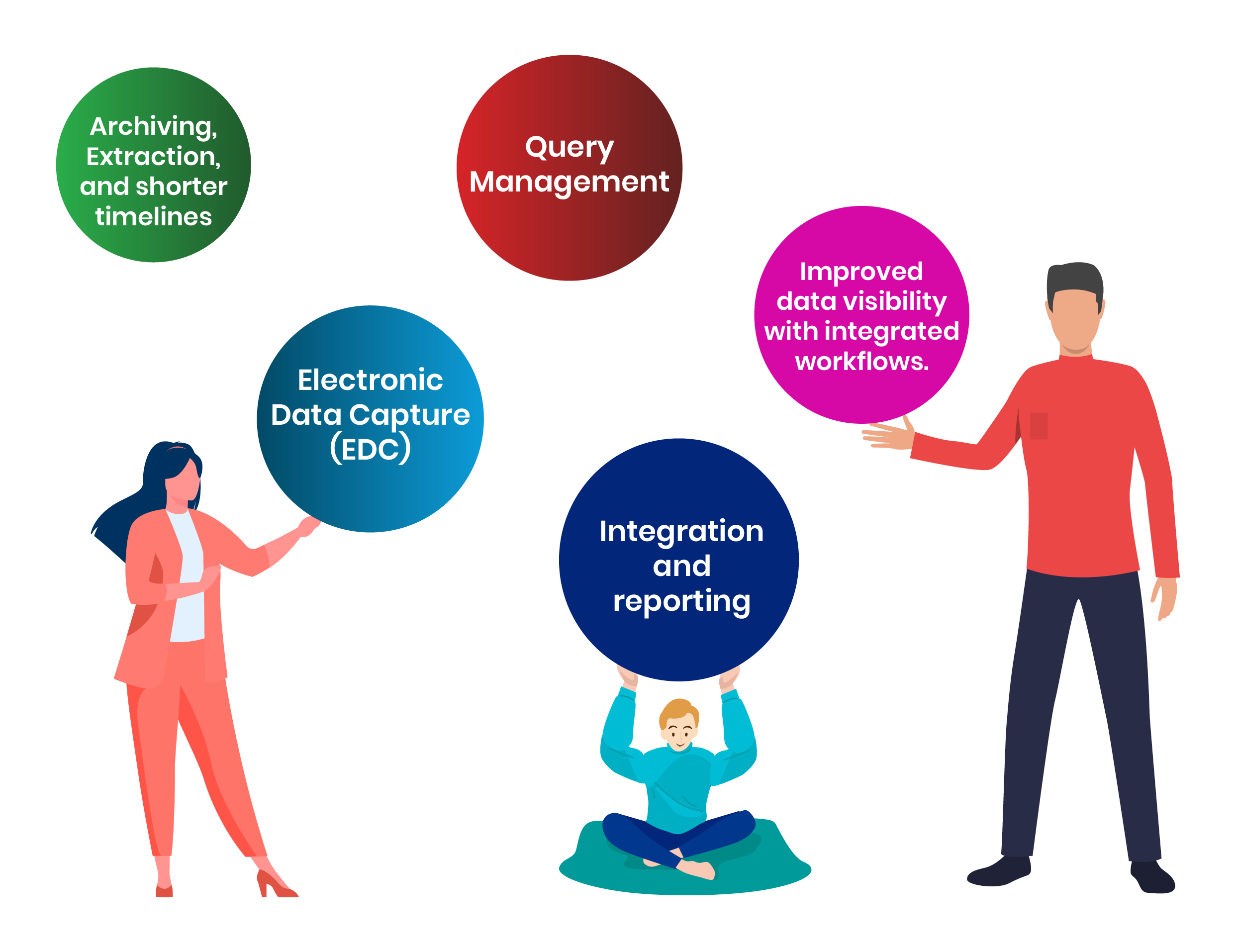
Clinical Data Collection
PureSoftware understands that the quality and integrity of the data is of primary importance. We ensure that data collection for clinical study analysis and regulatory submissions is done seamlessly. We leverage electronic data capture solutions including trial design and build services tailored to the specific needs of a clinical study following waterfall, agile, or a hybrid approach. Trials are designed to ensure there’s Integration of data from laboratories, IV/WRS / IRT; safety applications; and trial management applications.
Life Sciences Services & Solutions

Clinical Data
Collection
PureSoftware understands that the quality and integrity of the data are of primary importance.
know more
Clinical Data
Management
Clinical Data Management is a critical phase in clinical research, and data integrity is of paramount importance.
know more
Pharmacovigilance
The complexities involved in regulatory safety reporting necessitates proficiency to ensure quick access to intelligent data.
know more
Managed Cloud
Services
PureSoftware offers a spectrum of integrating applications and infrastructure services tailored to meet client-specific needs.
know more
Our Digital Capabilities for Life Sciences
We value partnering with life sciences organizations and develop technology-based solutions for them.
know moreWhy Choose PureSoftware?
- Enhanced operational efficiencies which can be measured through metrics.
- Realizable productivity enhancement through improved processes, and significant cost benefits.
- Data Management (CDM) & Biostatistics Team working together for better synergies.
- Full transparency in dealing and robust governance
We have some of the World’s Best Life Science Organizations relying on us.
You can become the next one.



